Chemical Effects of Electric current is a chapter in the Class 8 science book. We have shared accurate answers to all questions. We noticed that a lot of websites are sharing the wrong answers with the students.
An electric current runs all the appliances in our homes. Chemical substances contain ions which are important for the conduction of electric current. In this chapter, you will get to learn the concepts of cathode, anode, electric current, different chemicals that allow current flow. Then you will learn the concept of electroplating and other related concepts.
Chemical Effects of Electric Current NCERT Solutions
Q.1. Fill in the blanks:
(a) Most liquids that conduct electricity are solutions of __________ , __________ and __________.
(b) The passage of an electric current through a solution causes__________effect.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the __________terminal of the battery.
(d) The process of depositing a layer of any desired metal on another metallic object, by means of electricity, is culled __________.
Ans 1:
a) acid, bases and salts
b) chemical
c) negative
d) Electroplating
Question 2. When the free ends of a tester are dipped into a solution the magnetic needle shows deflection. Can you explain the reason?
Ans2 : When the free ends of tester are dipped into the solution the magnetic needle deflects because the circuit gets completed and current passes through it. This tells us that the solution we have taken is a good conductor of electricity.
Question 3: Name three liquids, which when tested in the manner shown in Mg. 14.1 may cause the magnetic needle to deflect
Ans 3: We can take lemon juice, tap water and HCl to test this. The needle will deflect in all these liquids.
Question 4: The bulb does not glow in the set up shown in Fig .14.2. List the possible reasons. Explain your answer.
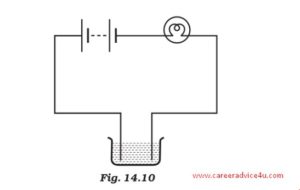
Ans 4: The following reasons can be responsible for this:-
a) The bulb might be fuse
b) There may be a break in the circuit. The circuit might not be completed
c) The solution taken might not be a good conductor of electricity
Question 5: A tester is used to check the conduction of electricity through two liquids, labeled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows very dimly for liquid B. You would conclude that:
(i) liquid A is a better conductor than liquid B.
(ii) liquid B is a better conductor than liquid A.
(iii) both liquids are equally conducting.
(iv) conducting properties of liquid cannot be compared in this arrangement
Ans 5: i) Liquid is A better conductor than liquid B
Question 6: Does pure water conduct electricity? If not what can we do to make it conducting?
Ans 6: Pure water does not conduct electricity because it does not contain ions. Ions are important for the conduction of electricity. We can use tap water or groundwater or can add salts to this water so that it conducts electricity.
Question 7: In case of a fire before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Ans 7: The firemen shut the main supply of the area because the water contain ions and can conduct electricity. If they do not turn off the mains then electrocution may happen and can lead to loss of life.
Question 8: A child staying in the coastal region tests the drinking water and also the sea water with his tester. He finds that the compass needle deflects more in the case of sea water. Can you explain the reason?
Ans 8: A child staying in the coastal region tests the drinking water and also the seawater with his tester. The seawater contains salt that may conduct electricity. So, the compass needle will deflect more in this case.
Question 9: Is it safe for the electrician to carry out electrical repairs outdoors during heavy down-pour? Explain.
Ans 9: No it is not safe to carry our electrical repairs during a heavy downpour. It can cause electric shocks. So,we should avoid any electrical repairs during rainfall.
Question 10: Paheli had heard that rain. water is as good as distilled water. So she collected some rain water in a clean glass tumbler and tested it using a tester. To her surprise she found that the compass needle showed deflection. What could be the reasons?
Ans 10: Rainwater is good conductor of electricity as it may contain impurities and salts. That’s the reason that the tester showed the deflection.
Question11: Prepare a list of objects around you that are electroplated
Ans 11: The objects that are electroplated in my surroundings are: Door handles, utensils in kitchen, almirah handles, ornaments and bath taps.
Question 12: The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from the impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?
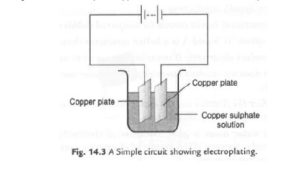
Ans 12: Impure copper rod should be connected to the positive terminal and the impure copper rod should be connected to the negative terminal of the battery. This allows the ions from impure copper to be released in the solutions. And Cu 2+ ions from the solution gets collected to pure one when an electric current is passed through it.
So, this was Deepak Kumar here to discuss Chemical Effects of Electrical Current NCERT Solutions. Don’t forget to comment if you need free guidance for your career. Stay connected to Careeradvice4u for more NCERT Solutions for Science and other subjects.
Latest posts by Deepak Kumar (see all)

Leave a Reply