
Acids Bases and Salts is a chapter in DAV Class 7 Science book. This chapter revolves around the discussion of acids, bases and their properties. It also discusses some common acids, bases and salts, foods that are acidic and basic nature etc.
DAV Class 7 Chapter 4 Solutions
A. Fill in the blanks.
1. Acids which are present in plants and animals are called ___________.
2. Bases taste ___________ and have a ___________ feel.
3. Acids turns the colour of blue litmus paper to ___________.
4. The products of neutralisation reaction are ___________ and ___________.
5. Salts of nitric acid (HNO3) are named as ___________.
6. Sodium acetate (CH3COONa) is a basic salt formed by the reaction of ___________ and ___________.
Ans A.
(1) organic acids
(2) bitter, soapy
(3) Red
(4) salt, water
(5) nitrate
(6) acetic acid ( CH3COOH), sodium hydroxide (NaOH)
Also Check: DAV Class 7 Science Heat Solutions
B. Match the following.
1. Lemon juice (a) Oxalic acid
2. Tamarind (b) Lactic acid
3. Vinegar (c) Citric acid
4. Red ants (d) Acetic acid
5. Sour milk (e) Tartaric acid
6. Guava (f) Formic acid
Ans B.
Lemon Juice – Citric Acid
Tamarind: Tartaric Acid
Vinegar: Acetic Acid
Red Ants: Formic Acid
Sour Milk: Lactic Acid
Guava: Oxalic Acid
C. Tick the correct option
Answers:-
1. bitter taste and a soapy feel
2. litmus
3. citric acid
4. nitric acid
5. salts
6. turmeric
7. sour and bitter
D. Answer the following questions in brief
1. What are mineral acids?
Acids that are prepared from minerals are called mineral acids. Examples: Hydrochloric acids, nitric Acid
Give two examples each of mineral acids and organic acids
Ans 2: Mineral Acids: Hydrochloric Acid, Boric Acid
Organic Acid: tartaric acid, Citric Acid
Name two substances that can be used as indicators
Turmeric and Litmus paper can be used as indicators
Write the meaning of the term ‘ Neutralization Reaction’
Neutralization reaction is a type of reaction in which an acid and base reacts
with each other to form a salt and water
Ex. HCl (Acid) + NaOH (Base) —> NaCl (Salt) + H2O
Question 5: Give any two properties of Salts.
Ans 5:
- Most of the Salts formed are soluble in water.
- Solution of salts in water act as a good conductor of electricity.
Question 6: Classify the following salt as acidic, basic and neutral. Also, write their names
Ans 6:
(a) Na3PO4
(b) K2CO3
(c) NH4NO3
Sodium Phosphate is a neutral salt
Potassium Carbonate is a basic salt
Ammonium Nitrate is an acidic salt
E. Answer the following questions
Question 1. All alkalies are bases but all bases are not alkalies. Justify this statement.
Alkalis is the term used for bases that are soluble in water. Bases that are not soluble in water can not be termed as Alkalis so we can say that all alkalis are bases but all bases can not be alkali.
Example: NaHCO3 (Baking Powder) is an alkali as it can dissolve in water. Aluminium hydroxide is insoluble in water so it can not be called an alkali.
Question 2: Suggest an activity that can help one to decide whether a given solution is acidic or basic in nature
Ans 2:
Take the solution in a beaker. Take litmus paper of both blue and red colour and dip them in the beaker containing the solution. If the blue colour litmus paper changes to red then it is an acid and i the red colour litmus paper changes to blue then it is a base in nature.
3. Write chemical equations for the following reactions:

Ans 3:
(a) Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O
(b) 2CH3COOH + Ca(OH)2 → Ca(CH3COO)2 + 2H2O
(c) HCl + NaOH → NaCl + H2O
(d) 2NH4OH + H2SO4 → (NH4)2SO4+ 2H2O
Also Check: Transportation in Plants and Animals Solutions DAV Class 7
Question 4: State the difference between neutral, acidic and basic Salts. Give one example of each.
Ans 4:
Neutral salt
Neutral salt is the one in which a strong acid reacts with a strong base.
No change in the colour when litmus paper is used.
Example: Sodium Phosphate ( Na3PO4)
Acidic Salt
Acidic salts are those in which a strong acid reacts with a weak base. Example: Ammonium nitrate: NH4NO3
Colour of blue litmus paper changes to red
Basic Salt
Basic salts are those that have a strong base and a weak acid:
Example: Potassium Carbonate:
K2CO3 has KOH as a base which is a strong base.
Question 5: Describe an activity that shows that solutions of salts in water conducts electricity.
Answer:
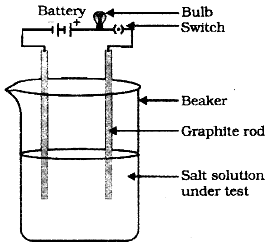
1) To show this thing you have to take a beaker and fill it half with water.
2) Take some common salt (NaCl) and dissolve it in this water. Take 2 graphite rods and connect them with the two terminals of a battery and a bulb as shown in the figure.
3) Bring these rods and allow them to dip in the beaker containing the solution in Step 1. When you do this the bulb will start glowing due to the flow of electric current. This clearly shows that a solution of sodium chloride can conduct electricity.
So, these were DAV Class 7 Science, Chapter 4 solutions. We have given answers to all the questions chapter Acids, Bases and Salts. If you have any kind of query, you can ask through the comments section below. Don’t forget to sign up for the form below to win free science Ebooks and other goodies. Stay tuned to Careeradvice4u.
Electric Current Its Effects Solutions | DAV Class 7

Leave a Reply